Emergency Response
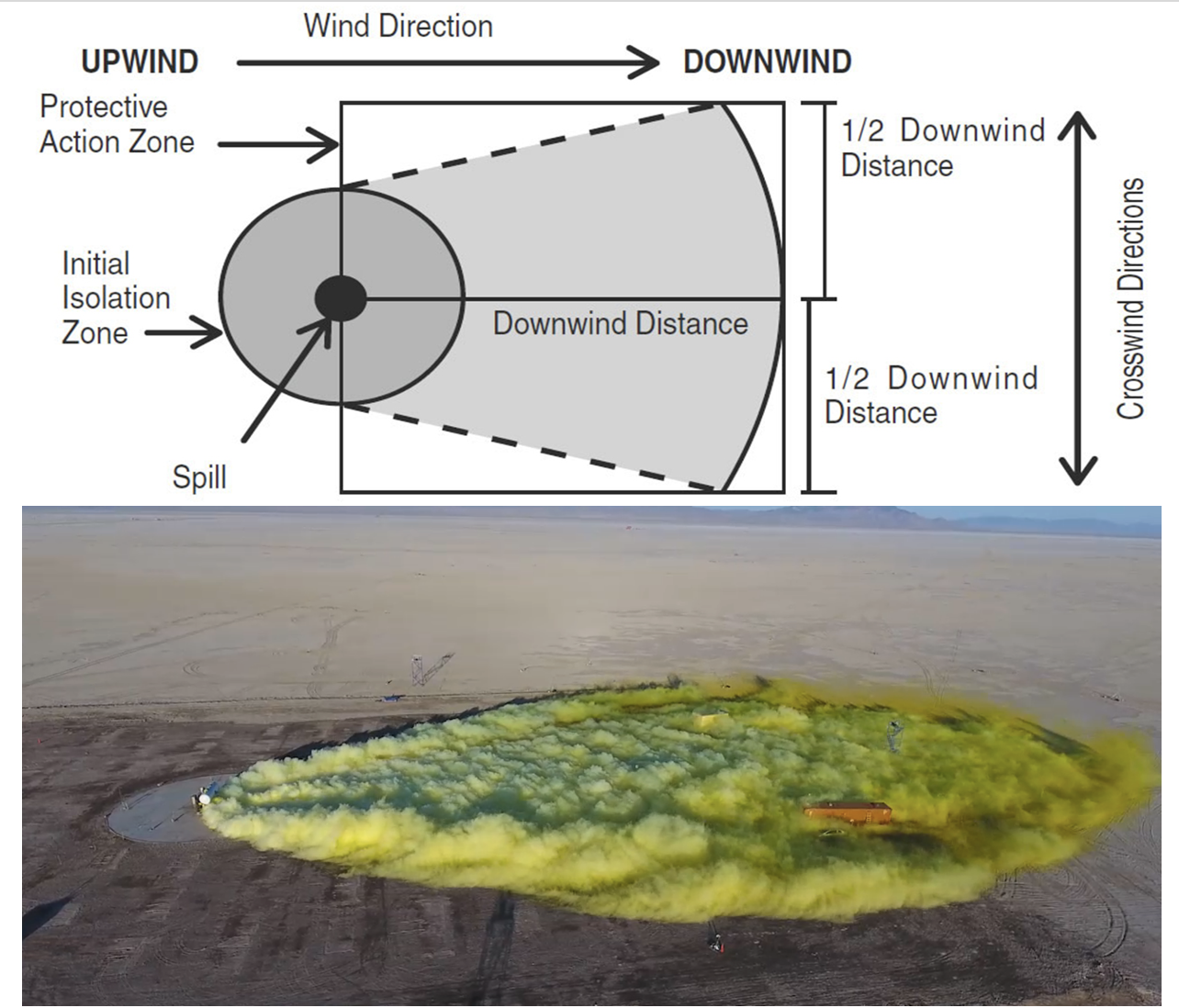
An image from my HAZMAT courses... People wonder why the ERG Protective Action Distance diagram is shaped like it is. This image validates the model...
NOTE: Cl2 release image is from the Jack Rabbit testing project Add new comment
Paragraph (e)(3) of the 1910.157 specifies that employers must subject each portable fire extinguisher
In addition, this provision requires employers to retain the inspection record for
and to make the record available to OSHA on request. This recordkeeping requirement assures workers and agency compliance officers that portable fire extinguishers located in the workplace will operate normally in case of fire; in addition, this requirement provides evidence to OSHA compliance officers during an inspection that the employer performed the required maintenance checks on the portable fire extinguishers.
This is the lens through which we should view EMERGENCY RESPONSE and our local FD as the "primary responders." Just because we have a local FD (paid full-time or volunteer) does not mean we have the emergency services we need! Far too often, our "assumptions" about the fire service's capabilities far outstretch their actual abilities. Regarding firefighting, rescue, and EMS, as well as response times, staffing, and equipment, we may be shocked at what our local FD can and can NOT provide. The FIRST STEP in Emergency Planning is sitting down with the FD leadership on an ANNUAL basis to analyze our needs and their abilities to provide those needs in a TIMELY manner. CLICK HERE for a great article at Fire Rescue 1
The last vehicle in the row was a vehicle carrying paint solvents - class 3 flammable liquids – which leaked onto the road due to the impact and immediately caught fire (0.5 sec). The fire engulfed the LPG tank quickly, initially only the front and then totally. The flames continued to burn the LPG tank, and about 7 minutes a BLEVE occurred. (see pictures 3,4 in Annex 1). Because of the energy released and the overpressure and heat generated, the road infrastructure collapsed, and the neighboring structures/buildings were strongly affected (see pictures 5, 6, and 7 in Annex 1). In the end, one person died (the driver of the tank vehicle), and 95 persons were injured (all were admitted to the hospitals near the area). Among the injured persons, some police officers and emergency teams rushed to the accident area. Their timely intervention allowed for clearing the area before the explosion, thus reducing damages and saving all the other people present near the scene of the accident. CLICK HERE for the UN Report
Every day of the year, billions of cubic feet of gas are stored, transported, and used without any problems or mishaps. This results from a conscientious, solid - effort toward safety within the gas industry. Even with this effort, from time to time, something goes wrong; either mechanical or human error occurs. When this happens, a hazard to man and his environment may evolve. Because of the laws of nature and the gas properties involved, this hazard can be analyzed and mapped out. This presentation will analyze these hazards and outline some actions that can be taken to nullify, reduce, or prevent the risk from becoming a disaster. To begin with, there are two (2) fundamental hazards:
Should something happen and the gas escape, we would now have a hazard outside of the container, which can be divided into either a
or
If the gas ignites upon escaping, we will have a fire situation. Our main concern will be heat exposure to people or property. The main objective is to prevent the exposure from being destroyed by the fire. The best way to accomplish this is to eliminate the heat source. THE ONLY WAY AN ESCAPING GAS FIRE SHOULD BE EXTINGUISHED IS BY STOPPING THE FLOW OF GAS The only exception to this rule is to affect an immediate rescue or to facilitate the immediate shutting off of the gas. If the gas cannot be shut off, the application of water on the exposure, in large quantities, will have to be continued until all the gas has burned out. The size of the line and the amount of water will vary depending on the size and scope of the fire, but remember; you will need to establish a constant water supply. It also will probably have to be in operation for an extended period. Applying water at a rate (GPM) greater than necessary is also better than not enough.
We will expand more on the specifics of fire conditions in a moment, but first, let's look at an outside-of-the-container gas hazard under a no-fire condition. We have four (4) distinct areas of concern, of which one or more could apply, depending on what gas is leaking. First, we could have a cryogenic hazard. The main hazard here is the extremely cold temperature of the liquid. Remember:
NO LIQUID CAN EXIST AT ATMOSPHERIC PRESSURE, AT A TEMPERATURE ABOVE ITS BOILING POINT
If there is liquid outside of its container, the temperature of the liquid will be at its boiling point. The boiling points of some common cryogenic gases are: Air ...........................-317.8°F Methane (NG) ........ -258.7°F Argon ..................... -302.6°F Nitrogen ................. -320.4°F Helium ................... -452.1°F Oxygen ................... -297.4°F Hydrogen ................ -423°F
The boiling points of some other gases not considered cryogenic but having similar cold hazards are: Acetylene ..................... -118°F Methyl chloride ............. -11°F Anhydrous ammonia ...... -28°F Methyl ether ...................-11°F Carbon oxysulfide ..........-58°F Propane ....................... -44°F Cyclopropane ............... -29°F Propylene ..................... -53°F Hydrogen sulfide .......... -76°F Propyne (mapp gas) ....... -10°F Isobutane ......................+11°F Anything that comes in contact with the liquid will immediately be frozen at these cold temperatures. Even the cold vapors close to the liquid could present a cold hazard. It would be best if you allowed no one to encounter any liquid with a boiling point below the freezing point of water (32° F), as living tissue is composed mainly of water and will freeze. Also, do not let anyone encounter any equipment displaying a frosting condition, such as piping or valving, as the temperature of these will be close to the same as the liquid it contains. If someone should come in contact, they must seek immediate medical attention. The second area of concern is the application of water and extinguishing agents to cryogenics. Remember, the temperature of any liquid gas outside of its container will be below the freezing point of water. Water from a municipal system generally has a temperature of about 50°F to 70°F, so any water application will cause an immediate BTU or heat input into the gas-liquid. This will cause an immediate increase in the rate of vaporization. Again, it becomes essential to identify the liquid and to consider some facts.
The next area of concern is the asphyxiant hazard, where, again, product identification becomes crucial. Asphyxiant means "to cause or undergo unconsciousness or death from lack of oxygen." Remember, humans need oxygen to survive. SCBA safeguards against this hazard, and proper ventilation eliminates it. You must also identify whether the gas is lighter or heavier than air so that it can be appropriately ventilated.
The third concern is whether the gas in question is combustible or noncombustible. Again, identifying the gas is very important, as a leak with no fire could result in either a flammable or a non-flammable gas. The dot marking for flammable gases is a red and white diamond with the four-digit United Nations identification number. There will be a number 2 in the diamond, the international numerical marking for gases. Do not get this mixed up with the NFPA 704 marking system, as flammable gases would have a number 4 in the red or top section. The D.O.T. marking for flammable gases is a red and white, diamond-shaped placard. The U.N. Identification number will be in the center of the placard. The hazard class number 2 will be in the bottom tip of the placard. If the gas is non-flammable, there is no combustion hazard except for oxygen, which is a non-flammable gas. As the concentration of oxygen increases above 21%, the ignition temperature of many combustibles is lowered. Also, things bum with much more intensity in oxygen-enriched atmospheres. Some examples of non-flammable gases are carbon dioxide, argon, nitrogen, etc. With flammable gases such as methane, ethane, propane, butane, acetylene, carbon monoxide, and others, the combustion explosion problem is a major concern. Any time a flammable gas is exposed to the atmosphere, where it can become mixed with oxygen, there is the potential for a combustion explosion. All that is needed is for it to reach a source of heat at or above the ignition temperature of the gas. The combustion explosion hazard can be divided into two (2) concerns. One concerns an explosion occurring inside a structure, and the other concerns an explosion outside of a structure. Remember, if you extinguish the fire and are not able to stop the flow of gas, it may eventually reach the same or another source of ignition and ignite again, in which case you will have the same or worse situation. If a combustion explosion occurs inside a building, the amount will depend on how much gas has accumulated before ignition. The more gas, the more disastrous the results; in fact, buildings have been leveled and civilians and firefighters killed. The objective is to prevent the combustion explosion from occurring, which, in most cases, is easier said than done. Some methods you may employ are stopping the gas flow, removing sources of ignition, or using fog streams to dilute or divert the gas from reaching known ignition sources. Whenever you are investigating a gas odor, an industrial gas incident, or an accident involving gas cylinders or tanks, a combustible gas indicator should be used to determine if there is a leak, especially where odorless gases are involved, and to determine if the level of concentration has reached the lower flammable limit. A combustible gas indicator is the only true way to tell. If you send firefighters into buildings to investigate gas leaks or incidents or ventilate a gas leak without first using a CGI, you are asking for disaster.
Always remember to PREPARE FOR THE WORST AND HOPE FOR THE BEST Preparation is the key to success.
The fourth area of concern regarding a no-fire outside container hazard is the toxic hazard. Once again, it becomes vitally important to identify what gas is escaping. Non-toxic gases such as CO2, Argon, Nitrogen, Methane, and Propane present no physiological or toxic hazard to health. However, toxic gases such as Carbon Monoxide, Acrolein, Ammonia, Chlorine, Phosphate, and others can be deadly when inhaled, even in small amounts. Some of these gases are flammable, and inhaling their smoke can be just as deadly. As a safety procedure, SCBA and full protective clothing must be worn, and in some cases, as with ammonia, unique clothing must be worn as some toxic gases can be absorbed directly through the skin. It would be best if you kept all personnel out of the area unless they must be there and then use only the personnel necessary and no more. You must see that those persons are adequately protected. The adage, "Do something even if it's wrong" could kill responders. You must know what you are dealing with, the potential hazard, and how to alter the course of events that are causing the hazard.
Let us now move to the INSIDE CONTAINER HAZARD. Under normal conditions, the only hazard is the gas's attempt to escape. The major hazard is going to be from an exposure fire. The fire may involve combustibles around the gas containers, such as a building, grass, wood, vehicles, etc. This exposure fire is causing the container and its contents, either liquid or vapor, to be heated. According to Charles' Law, (see Basic and Combined Gas Laws section), raising the gas temperature causes the gas to expand and the pressure to rise. If the pressure rises beyond the design strength of the container, the container will fail. We must take strict precautions to ensure that oxygen is never mixed with flammable gas in its container. Therefore, a fire can never start inside a container. The surest method of eliminating the hazard is to extinguish the fire. Extinguishing the fire may not be possible or even advisable, as would be the case if the exposed fire were from escaping gas. Then, you must treat the tank or container as an exposure and deluge it with water to keep the temperature down. The container you are trying to cool will be one of two (2) types:
With an insulated container, you want to be sure the exposed fire is heating the tank. Applying water to a tank containing a cryogenic will, in effect, attempt to warm the contents, and the only reason you would apply water would be to absorb the extreme heat of the fire. If you are fortunate to be facing an insulated container, you will have one thing going for you: the insulation will slow down the transfer of heat from the fire to the contents or even to the pressure vessel. This is not the case with an uninsulated container. There will only be a thin layer of steel or aluminum between the fire and the contents. If you do nothing and your actions are insufficient to keep the container or its contents at a safe temperature, the situation will worsen, and you may not even realize it. The contents of the container may be either a compressed gas or a liquefied gas. If it is a compressed gas, the pressure will rise, and hopefully, the relief valve will open and prevent the container from failing. However, the container could fail due to the extreme heat of the fire. In any case, there will be a release of gas. Remember, some poisonous gases are not allowed to have pressure relief valves. If the contents are a liquefied gas, the relief valve may still operate to relieve the pressure. However, if the container fails, the released gas will be much more destructive and disastrous than the compressed gas release. The failure even has a name, BLEVE, which will be discussed in the next part of the program. To summarize, let's go back to the main hazard points. The gas hazard will be either inside or outside of the container. If outside the container, there will be either no fire or a fire condition. If a no-fire situation there will be one of four hazards:
If it is a combustion hazard, it will be either indoors or outdoors. If it is an inside container hazard, the problem will usually be from an exposure fire. The gas will be either in an uninsulated or an insulated container. An insulated container buys you a bit more time. The gas will either be a compressed gas, the result of which will be a gas release, or it will be a liquefied gas, in which case the result could be a BLEVE.
Source: The National Fire Academy (Document seems to be out of publication)
BOYLES LAW: When the temperature is kept constant, the volume of a gas is inversely proportional to the pressure upon it. CHARLES LAW: When the external pressure is kept constant, the volume of a gas is directly proportional to its absolute temperature. |
Partner Organizations I am proud to announce that The Chlorine Institute and SAFTENG have extended our"Partners in Safety" agreement for another year (2024) CI Members, send me an e-mail to request your FREE SAFTENG membership
Member Associations
|
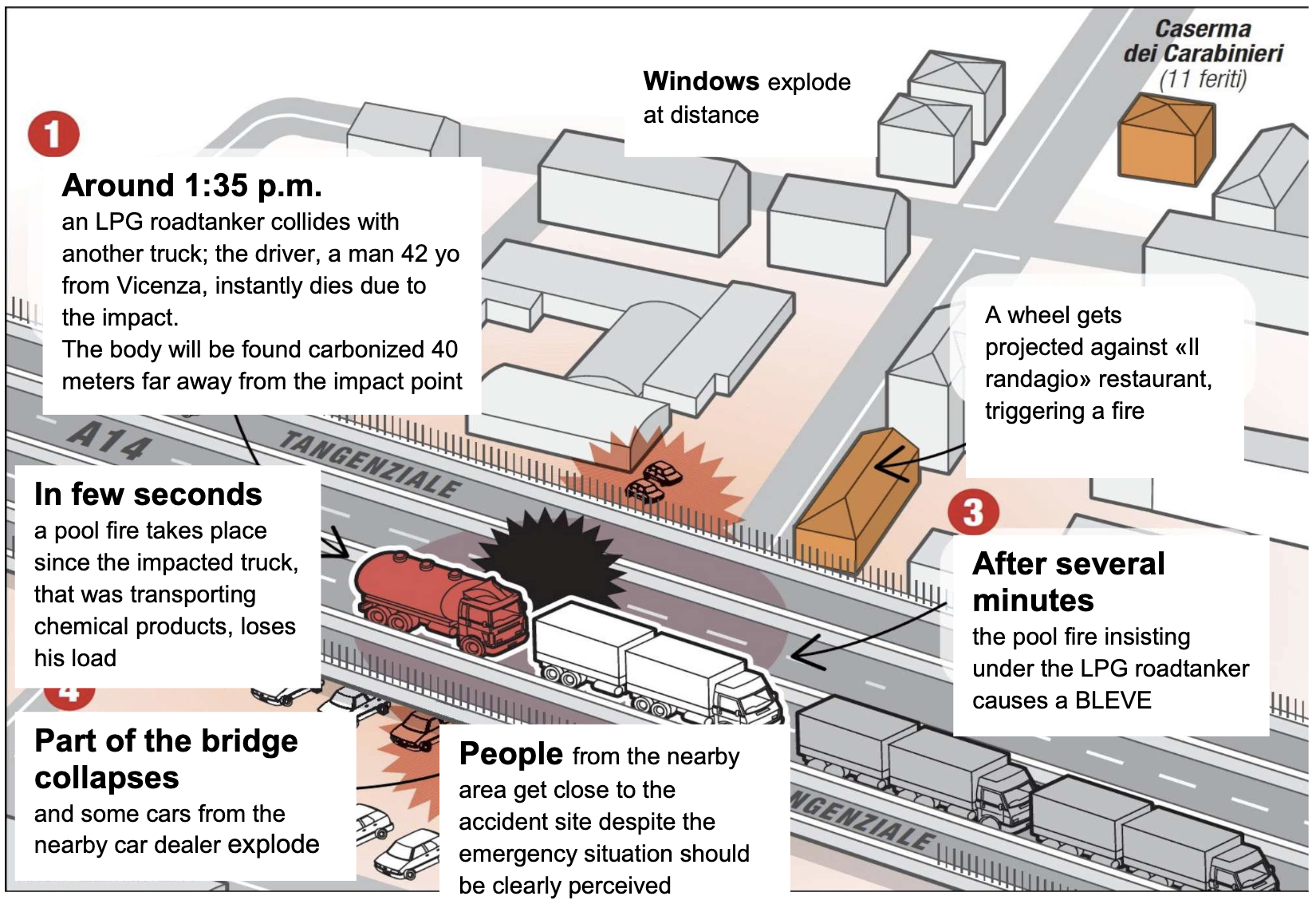 On August 6, 2018, at 1:35 pm, an event occurred at Km 4+300 of the road connection between Motorway A1 and Motorway A14 near Bologna (see picture 1 in Annex 1). The section of the road flows over an elevated structure above the urban road network below. Among the vehicles involved in the accidents, there were two ADR vehicles. A tractor with a semitrailer with an LPG class 2 tank that was traveling in the direction north, for reasons to be clarified, violently hit the column of vehicles stationary in the right lane. The driver of the LPG tank vehicle died immediately due to the impact (see picture 2 in Annex 1).
On August 6, 2018, at 1:35 pm, an event occurred at Km 4+300 of the road connection between Motorway A1 and Motorway A14 near Bologna (see picture 1 in Annex 1). The section of the road flows over an elevated structure above the urban road network below. Among the vehicles involved in the accidents, there were two ADR vehicles. A tractor with a semitrailer with an LPG class 2 tank that was traveling in the direction north, for reasons to be clarified, violently hit the column of vehicles stationary in the right lane. The driver of the LPG tank vehicle died immediately due to the impact (see picture 2 in Annex 1).












